药品详细
Ranitidine(雷尼替丁)
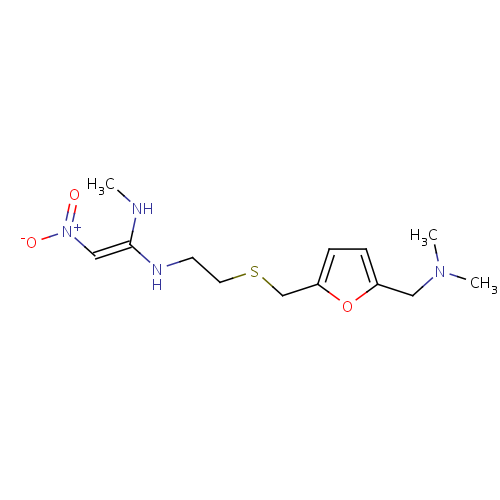
化学结构式图
中文名
雷尼替丁
英文名
Ranitidine
分子式
C13H22N4O3S
化学名
dimethyl[(5-{[(2-{[(E)-1-(methylamino)-2-nitroethenyl]amino}ethyl)sulfanyl]methyl}furan-2-yl)methyl]amine
分子量
Average: 314.404
Monoisotopic: 314.14126128
Monoisotopic: 314.14126128
CAS号
66357-35-5
ATC分类
A02B 未知;A02B 未知
药物类型
small molecule
阶段
approved
商品名
Alquen;Alter-H2;Alvidina;Apo-Ranitidin;Artomil;Azuranit;Coralen;Digestosan;Ergan;Esofex;Fendibina;Gastrial;Gastridina;Gastrolav;Gastrosedol;Kuracid;Label;Lake;Logat;Melfax;Microtid;Mideran;Neugal;Noctone;Noktome;Normon;Novo-Radinine;Nu-Ranit;Pep-Rani;Ptinolin;Quadrin;Quantor;Radin;RAN;Ran H2;Ran Lich;Rani 2;Rani AbZ;Rani-BASF;Rani-nerton;Rani-Puren;Rani-Q;Rani-Sanorania;Raniben;Raniberl;Raniberta;Ranibloc;Ranic;Ranicux;Ranidil;Ranidin;Ranidine;Ranidura;Ranifur;Ranigasan;Ranigast;Ranigen;Ranilonga;Ranimerck;Raniogas;Raniplex;Ranisan;Ranitab;Ranitic;Ranitidin;Ranitidin 1A Pharma;Ranitidin AL;Ranitidin Arcana;Ranitidin Atid;Ranitidin AWD;Ranitidin Basics;Ranitidin Duncan;Ranitidin Dyna;Ranitidin Helvepharm;Ranitidin Heumann;Ranitidin Hexal;Ranitidin Merck;Ranitidin Millet;Ranitidin NM;Ranitidin Normon;Ranitidin PB;Ranitidin Stada;Ranitidin von ct;Ranitidin-Cophar;Ranitidin-Isis;Ranitidin-ratiopharm;Ranitidina predilu Grif;Ranitidina Tamarang;Ranitiget;Ranitin;Ranitine;Ranobel;Rantacid;Ranuber;Raticina;Regalil;Renatac;Rozon;Rubiulcer;Santanol;Serviradine;Sostril;Tanidina;Taural;Terposen;Toriol;Trigger;Ulcecur;Ulcex;Ulcirex;Ulcodin;Ulcolind Rani;Ulsaven;Ultidine;Viserul;Zandid;Zantac;Zantac 150;Zantac 75;Zantac In Plastic Container;Zantarac;Zantic;
同义名
Ranitidine Base;Ranitidine HCL;Ranitidine hydrochloride;Rantidine HCL;
基本介绍
A non-imidazole blocker of those histamine receptors that mediate gastric secretion (H2 receptors). It is used to treat gastrointestinal ulcers. [PubChem]
生产厂家
- Alpharma us pharmaceuticals division
- Amneal pharmaceuticals
- Amneal pharmaceuticals ny llc
- Apotex inc
- Aurobindo pharma usa inc
- Bedford laboratories div ben venue laboratories inc
- Ben venue laboratories inc
- Boehringer ingelheim
- Boehringer ingelheim corp
- Boehringer ingelheim pharmaceuticals inc
- Contract pharmacal corp
- Cypress pharmaceutical inc
- Dr reddys laboratories inc
- Dr reddys laboratories ltd
- Genpharm inc
- Glaxosmithkline
- Glenmark generics inc usa
- Ivax pharmaceuticals inc sub teva pharmaceuticals usa
- L perrigo co
- Mylan pharmaceuticals inc
- Par pharmaceutical inc
- Pharmaceutical assoc inc div beach products
- Ranbaxy laboratories ltd
- Ranbaxy pharmaceuticals inc
- Sandoz inc
- Teva pharmaceuticals usa inc
- Torpharm inc
- Vintage pharmaceuticals inc
- Watson laboratories inc
- Wockhardt americas inc
- Wockhardt ltd
封装厂家
- Actavis Group
- Advanced Pharmaceutical Services Inc.
- Aidarex Pharmacuticals LLC
- Amerisource Health Services Corp.
- Amneal Pharmaceuticals
- Apotex Inc.
- Apotheca Inc.
- Apothecon
- AQ Pharmaceuticals Inc.
- A-S Medication Solutions LLC
- Banner Pharmacaps Inc.
- Bedford Labs
- Ben Venue Laboratories Inc.
- Blenheim Pharmacal
- Boehringer Ingelheim Ltd.
- Bryant Ranch Prepack
- Cardinal Health
- Central Texas Community Health Centers
- Chain Drug
- Cobalt Pharmaceuticals Inc.
- Comprehensive Consultant Services Inc.
- Corepharma LLC
- Coupler Enterprises Inc.
- CVS Pharmacy
- Dept Health Central Pharmacy
- DHHS Program Support Center Supply Service Center
- Direct Dispensing Inc.
- Dispensing Solutions
- Diversified Healthcare Services Inc.
- Doctor Reddys Laboratories Ltd.
- DSM Corp.
- Fusion Pharmaceuticals LLC
- Genpharm LP
- GlaxoSmithKline Inc.
- Glenmark Generics Ltd.
- Golden State Medical Supply Inc.
- H.J. Harkins Co. Inc.
- Heartland Repack Services LLC
- Hospira Inc.
- Innovative Manufacturing and Distribution Services Inc.
- Innoviant Pharmacy Inc.
- Ivax Pharmaceuticals
- Kaiser Foundation Hospital
- Keltman Pharmaceuticals Inc.
- Lake Erie Medical and Surgical Supply
- Liberty Pharmaceuticals
- Major Pharmaceuticals
- Mckesson Corp.
- Medisca Inc.
- Medvantx Inc.
- Murfreesboro Pharmaceutical Nursing Supply
- Mylan
- Novopharm Ltd.
- Nucare Pharmaceuticals Inc.
- Ohm Laboratories Inc.
- Palmetto Pharmaceuticals Inc.
- Par Pharmaceuticals
- Patheon Inc.
- PCA LLC
- PD-Rx Pharmaceuticals Inc.
- Penn Labs
- Perrigo Co.
- Pharmaceutical Association
- Pharmaceutical Packaging Center
- Pharmedix
- Physicians Total Care Inc.
- Precision Dose Inc.
- Preferred Pharmaceuticals Inc.
- Prepackage Specialists
- Prepak Systems Inc.
- Prescription Dispensing Service Inc.
- Prx Pharmaceuticals
- Ranbaxy Laboratories
- Rebel Distributors Corp.
- Redpharm Drug
- Remedy Repack
- Resource Optimization and Innovation LLC
- Roxane Labs
- Sandhills Packaging Inc.
- Sandoz
- Shasun Chemicals & Drugs Ltd.
- Southwood Pharmaceuticals
- St Mary's Medical Park Pharmacy
- Sunmark
- Teva Pharmaceutical Industries Ltd.
- Torpharm Inc.
- Tya Pharmaceuticals
- UDL Laboratories
- Va Cmop Dallas
- Vangard Labs Inc.
- Vistapharm Inc.
- Walgreen Co.
- Watson Pharmaceuticals
- Wockhardt Ltd.
- Xactdose Inc.
参考
| Synthesis Reference | Not Available |
| General Reference | Not Available |
剂型
规格
化合物类型
| Type | small molecule |
| Classes |
|
| Substructures |
|
适应症
gastric acid RELATED DISORDERS 中和胃酸;
药理
| Indication | Used in the treatment of peptic ulcer disease (PUD), dyspepsia, stress ulcer prophylaxis, and gastroesophageal reflux disease (GERD). | ||||||
| Pharmacodynamics | Ranitidine is a histamine H2-receptor antagonist similar to cimetidine and famotidine. An H2-receptor antagonist, often shortened to H2 antagonist, is a drug used to block the action of histamine on parietal cells in the stomach, decreasing acid production by these cells. These drugs are used in the treatment of dyspepsia, however their use has waned since the advent of the more effective proton pump inhibitors. Like the H1-antihistamines, the H2 antagonists are inverse agonists rather than true receptor antagonists. | ||||||
| Mechanism of action | The H2 antagonists are competitive inhibitors of histamine at the parietal cell H2 receptor. They suppress the normal secretion of acid by parietal cells and the meal-stimulated secretion of acid. They accomplish this by two mechanisms: histamine released by ECL cells in the stomach is blocked from binding on parietal cell H2 receptors which stimulate acid secretion, and other substances that promote acid secretion (such as gastrin and acetylcholine) have a reduced effect on parietal cells when the H2 receptors are blocked. | ||||||
| Absorption | Approximately 50% bioavailability orally. | ||||||
| Volume of distribution |
|
||||||
| Protein binding | 15% | ||||||
| Metabolism |
Hepatic. Ranitidine is metabolized to the N-oxide, S-oxide, and N-desmethyl metabolites, accounting for approximately 4%, 1%, and 1% of the dose, respectively.
|
||||||
| Route of elimination | The principal route of excretion is the urine (active tubular excretion, renal clearance 410mL/min), with approximately 30% of the orally administered dose collected in the urine as unchanged drug in 24 hours. | ||||||
| Half life | 2.8-3.1 hours | ||||||
| Clearance |
|
||||||
| Toxicity | LD50=77mg/kg (orally in mice). Symptoms of overdose include muscular tremors, vomiting, and rapid respiration. | ||||||
| Affected organisms |
|
||||||
| Pathways |
|
理化性质
| Properties | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | |||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
|||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
|||||||||||||||||||||||||||||||||||||||
药物相互作用
| Drug | Interaction |
|---|---|
| Acenocoumarol | Ranitidine may increase the anticoagulant effect of acenocoumarol. (Conflicting evidence) |
| Anisindione | Ranitidine may increase the anticoagulant effect of anisindione. (Conflicting evidence) |
| Atazanavir | Ranitidine may decrease the levels/effects of atazanavir. |
| Cefditoren | H2-Antagonists such as ranitidine may decrease the serum concentration of cefditoren. Cefditoren prescribing information recommends to avoid concomitant use with H2-antagonists (eg, famotidine, ranitidine) and antacids as well. Consider alternative methods to minimize/control acid reflux (eg, diet modification) or alternative antimicrobial therapy if use of H2-antagonists can not be avoided. |
| Dabrafenib | H2-receptor antagonists may alter the solubility of dabrafenib and reduce its bioavailability. |
| Dasatinib | Ranitidine may decrease the serum level of dasatinib. |
| Dicumarol | Ranitidine may increase the anticoagulant effect of dicumarol. (Conflicting evidence) |
| Itraconazole | The H2-receptor antagonist, ranitidine, may decrease the absorption of itraconazole. |
| Ketoconazole | The H2-receptor antagonist, ranitidine, may decrease the absorption of ketoconazole. |
| Procainamide | The histamine H2-receptor antagonist, ranitidine, may increase the effect of procainamide. |
| Rilpivirine | Histamine-2 receptor antagonists increase gastric pH which causes a decrease in the exposure of rilpivirine thus reducing efficacy. |
| Tolazoline | Anticipated loss of efficacy of tolazoline |
| Vismodegib | Vismodegib serum concentrations may be decreased by histamine 2 receptor antagonists such as ranitidine. |
| Warfarin | Ranitidine may increase the anticoagulant effect of warfarin. (Conflicting evidence) |
食物相互作用
- Avoid alcohol.
- Avoid excessive quantities of coffee or tea (Caffeine).
- Avoid milk, calcium containing dairy products, iron, antacids, or aluminum salts 2 hours before or 6 hours after using antacids while on this medication.
- Take without regard to meals.
