药品详细
Irinotecan (伊立替康 )
化学结构式图
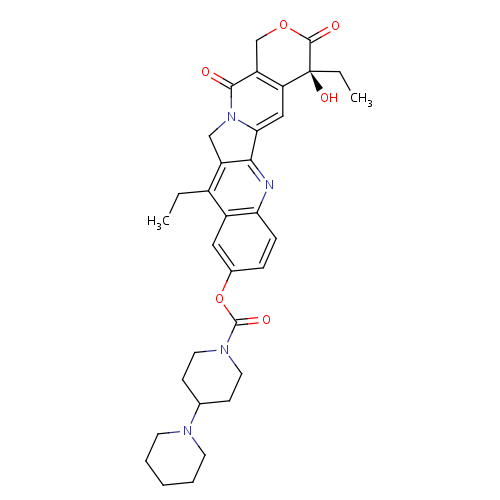
中文名
伊立替康
英文名
Irinotecan
分子式
Not Available
化学名
(19S)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-7-yl 4-(piperidin-1-yl)piperidine-1-carboxylate
分子量
Average: 586.678
Monoisotopic: 586.279134968
Monoisotopic: 586.279134968
CAS号
100286-90-6
ATC分类
L01X 其它抗肿瘤药
药物类型
small molecule
阶段
商品名
Camptosar;CP0;IRINOTECAN, CPT-11;
同义名
irinotecan;Irinotecan Hcl;Irinotecan Hydrochloride;Irinotecan Hydrochloride Trihydrate;Irinotecanum [INN-Latin];
基本介绍
Irinotecan is an antineoplastic enzyme inhibitor primarily used in the treatment of colorectal cancer. It is a derivative of camptothecin that inhibits the action of topoisomerase I. Irinotecan prevents religation of the DNA strand by binding to topoisomerase I-DNA complex, and causes double-strand DNA breakage and cell death.
生产厂家
- Accord healthcare inc
- Actavis totowa llc
- Akorn inc
- App pharmaceuticals
- Bedford laboratories
- Dr reddys laboratories ltd
- Ebewe pharma ges mbh nfg kg
- Fresenius kabi oncology plc
- Hospira inc
- Pfizer inc
- Pharmaforce inc
- Pliva lachema as
- Sandoz inc
- Sun pharma global inc
- Teva parenteral medicines inc
- Watson laboratories inc
封装厂家
参考
| Synthesis Reference | Not Available |
| General Reference |
|
剂型
| Form | Route | Strength |
|---|---|---|
| Solution | Intravenous |
规格
| Unit description | Cost | Unit |
|---|---|---|
| Irinotecan hcl 40 mg/2 ml inj | 138.07 USD | ml |
| Camptosar 20 mg/ml vial | 36.0 USD | ml |
| Irinotecan hcl 40 mg/2 ml vial | 22.2 USD | ml |
化合物类型
| Type | small molecule |
| Classes |
|
| Substructures |
|
适应症
Cancer 癌症;
药理
| Indication | For the treatment of metastatic colorectal cancer (first-line therapy when administered with 5-fluorouracil and leucovorin). Also used in combination with cisplatin for the treatment of extensive small cell lung cancer. Irinotecan is currently under investigation for the treatment of metastatic or recurrent cervical cancer. | ||||||
| Pharmacodynamics | Irinotecan is an antineoplastic enzyme inhibitor primarily used in the treatment of colorectal cancer. Irinotecan is a semisynthetic derivative of camptothecin. Camptothecins interact specifically with topoisomerase I, an enzyme in the cell nucleus that regulates DNA topology and facilitates nuclear processes such as DNA replication, recombination, and repair. During these processes, topoisomerase I relieves torsional strain in DNA by inducing reversible single-strand breaks, allowing single DNA strands to pass through the break. The 3'-DNA terminus of the broken DNA strands bind covalently with the topoisomerase enzyme to form a catalytic intermediate called a cleavable complex. After the DNA is sufficiently relaxed and the strand passage reaction is complete, DNA topoisomerase reattaches the broken DNA strands to form the chemically unaltered topoisomers that allow transcription to proceed. Irinotecan and its active metabolite SN-38 bind to the topoisomerase I-DNA complex and prevent religation of these single-strand breaks. Current research suggests that the cytotoxicity of irinotecan is due to double-strand DNA damage produced during DNA synthesis when replication enzymes interact with the ternary complex formed by topoisomerase I, DNA, and either Irinotecan or SN-38. Mammalian cells cannot efficiently repair these double-strand breaks. The precise contribution of SN-38 to the activity of irinotecan in humans is not known. Irinotecan is cell cycle phase-specific (S-phase). | ||||||
| Mechanism of action | Irinotecan inhibits the action of topoisomerase I. Irinotecan prevents religation of the DNA strand by binding to topoisomerase I-DNA complex. The formation of this ternary complex interferes with the moving replication fork, which induces replication arrest and lethal double-stranded breaks in DNA. As a result, DNA damage is not efficiently repaired and apoptosis (programmed cell death) occurs. | ||||||
| Absorption | 100% | ||||||
| Volume of distribution | Not Available | ||||||
| Protein binding | 30%-68% | ||||||
| Metabolism |
Hepatic |
||||||
| Route of elimination | The cumulative biliary and urinary excretion of irinotecan and its metabolites (SN-38 and SN-38 glucuronide) over a period of 48 hours following administration of irinotecan in two patients ranged from approximately 25% (100 mg/m2) to 50% (300 mg/m2). | ||||||
| Half life | 6-12 hours | ||||||
| Clearance | Not Available | ||||||
| Toxicity | Gastrointestinal complications, such as nausea, vomiting, abdominal cramping, diarrhea, and infection. | ||||||
| Affected organisms |
|
||||||
| Pathways |
|
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||
| Melting point | 222-223 oC | ||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
||||||||||||||||||||||||||||||||||||
药物相互作用
| Drug | Interaction |
|---|---|
| Aprepitant | Aprepitant may change levels of the chemotherapy agent, irinotecan. |
| Atazanavir | Increases levels/effect of irinotecan |
| Fosphenytoin | The hydantoin decreases the effect of irinotecan |
| Ketoconazole | Ketoconazole increases the effect and toxicity of irinotecan |
| Phenytoin | The hydantoin decreases the effect of irinotecan |
| Telithromycin | Telithromycin may reduce clearance of Irinotecan. Consider alternate therapy or monitor for changes in the therapeutic/adverse effects of Irinotecan if Telithromycin is initiated, discontinued or dose changed. |
| Thiotepa | Thiotepa, a strong CYP2B6 inhibitor, may decrease the metabolism and clearance of Irinotecan, a CYP2B6 substrate. Consider alternate therapy or monitor for changes in the therapeutic and adverse effects of Irinotecan if Thiotepa is initiated, discontinued or dose changed. |
| Trastuzumab | Trastuzumab may increase the risk of neutropenia and anemia. Monitor closely for signs and symptoms of adverse events. |
| Voriconazole | Voriconazole, a strong CYP3A4 inhibitor, may increase the serum concentration of irinotecan by decreasing its metabolism. Monitor for changes in the therapeutic and adverse effects of irinotecan if voriconazole is initiated, discontinued or dose changed. |
食物相互作用
Not Available
