药品详细
Glimepiride (格列美脲 )
化学结构式图
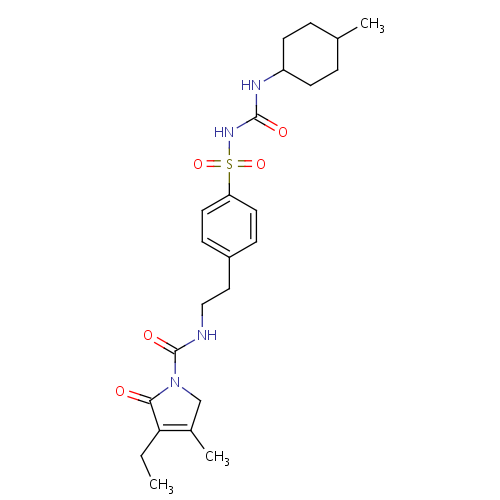
中文名
格列美脲
英文名
Glimepiride
分子式
N/A
化学名
3-ethyl-4-methyl-N-{2-[4-({[(4-methylcyclohexyl)carbamoyl]amino}sulfonyl)phenyl]ethyl}-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxamide
分子量
Average: 490.616
Monoisotopic: 490.224990908
Monoisotopic: 490.224990908
CAS号
93479-97-1
ATC分类
A10B Oral Blood Glucose Lowering Drugs, Excl. Insulins
药物类型
small molecule
阶段
商品名
Amarel;Amaryl;Endial;Novo-glimepiride;PMS-glimepiride;Ratio-glimepiride;Sandoz glimepiride;
同义名
Glimepirid;Glimepirida;Glimepiridum;Glimepride;
基本介绍
Glimepiride is the first III generation sulphonyl urea it is a very potent sulphonyl urea with long duration of action.
生产厂家
封装厂家
参考
| Synthesis Reference | Not Available |
| General Reference | Not Available |
剂型
| Form | Route | Strength |
|---|---|---|
| Tablet | Oral |
规格
| Unit description | Cost | Unit |
|---|---|---|
| Amaryl 4 mg tablet | 2.11 USD | tablet |
| Amaryl 2 mg tablet | 1.25 USD | tablet |
| Glimepiride 4 mg tablet | 1.25 USD | tablet |
| Amaryl 1 mg tablet | 0.88 USD | tablet |
| Glimepiride 2 mg tablet | 0.67 USD | tablet |
| Glimepiride 1 mg tablet | 0.42 USD | tablet |
化合物类型
| Type | small molecule |
| Classes |
|
| Substructures |
|
适应症
;Diabetes 糖尿病;
药理
| Indication | For concomitant use with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus. |
| Pharmacodynamics | Glimepiride, like glyburide and glipizide, is a "second-generation" sulfonylurea agents. Glimepiride is used with diet to lower blood glucose by increasing the secretion of insulin from pancreas and increasing the sensitivity of peripheral tissues to insulin. |
| Mechanism of action | The mechanism of action of glimepiride in lowering blood glucose appears to be dependent on stimulating the release of insulin from functioning pancreatic beta cells, and increasing sensitivity of peripheral tissues to insulin. Glimepiride likely binds to ATP-sensitive potassium channel receptors on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Membrane depolarization stimulates calcium ion influx through voltage-sensitive calcium channels. This increase in intracellular calcium ion concentration induces the secretion of insulin. |
| Absorption | Completely (100%) absorbed following oral administration. |
| Volume of distribution |
|
| Protein binding | Over 99.5% bound to plasma protein. |
| Metabolism |
Hepatic. Following either an intravenous or oral dose, glimepiride is completely metabolized by oxidative biotransformation to a major metabolite, cyclohexyl hydroxymethyl derivative (M1), via the hepatic cytochrome P450 II C9 subsystem. M1 is further metabolized to the carboxyl derivative (M2) by one or several cytosolic enzymes. M1, but not M2, possessed approximately one third of the pharmacologic activity of its parent in an animal model. However, whether the glucose-lowering effect of M1 is clinically significant is not clear. |
| Route of elimination | Not Available |
| Half life | Approximately 5 hours following single dose. |
| Clearance |
|
| Toxicity | Severe hypoglycemic reactions with coma, seizure, or other neurological impairment. |
| Affected organisms |
|
| Pathways | Not Available |
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||
| Melting point | 207 oC | ||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||
药物相互作用
| Drug | Interaction |
|---|---|
| Cyclosporine | The sulfonylurea, glimepiride, may increase the effect of cyclosporine. |
| Gemfibrozil | Gemfibrozil increases the effect and toxicity of rosiglitazone/pioglitazone |
| Glucosamine | Possible hyperglycemia |
| Ketoconazole | Ketoconazole increases the effect of rosiglitazone |
| Rifampin | Rifampin may decrease the effect of sulfonylurea, glimepiride. |
| Somatropin recombinant | Somatropin may antagonize the hypoglycemic effect of glimepiride. Monitor for changes in fasting and postprandial blood sugars. |
| Tolbutamide | Tolbutamide, a strong CYP2C9 inhibitor, may decrease the metabolism and clearance of Glimepiride. Consider alternate therapy or monitor for changes in Glimepiride therapeutic and adverse effects if Tolbutamide is initiated, discontinued or dose changed. |
食物相互作用
- Avoid alcohol.
- Even though food reduces product absorption, the manufacturer recommends taking the product with the first meal of the day.