药品详细
Sucralfate(硫糖铝)
化学结构式图
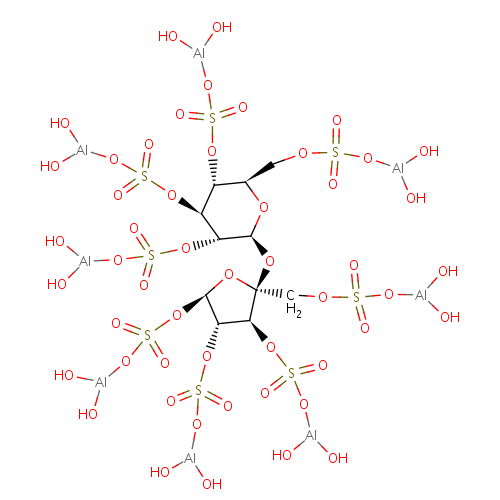
中文名
硫糖铝
英文名
Sucralfate
分子式
C11H28Al8O51S8
化学名
[({[(2S,3R,4S,5R,6R)-4,5-bis({[(dihydroxyalumanyl)oxy]sulfonyl}oxy)-6-[({[(dihydroxyalumanyl)oxy]sulfonyl}oxy)methyl]-2-{[(2R,3S,4S,5R)-3,4,5-tris({[(dihydroxyalumanyl)oxy]sulfonyl}oxy)-2-[({[(dihydroxyalumanyl)oxy]sulfonyl}oxy)methyl]oxolan-2-yl]oxy}oxan-3-yl]oxy}sulfonyl)oxy]alumanediol
分子量
Average: 1448.682
Monoisotopic: 1447.588619666
Monoisotopic: 1447.588619666
CAS号
54182-58-0
ATC分类
A02B 未知
药物类型
small molecule
阶段
approved
商品名
Antepsin;Apo-sucralfate;Carafate;Sucramal;Sulcrate;Sulcrate Suspension Plus;Ulcar;Ulcerban;Ulcerlmin;Ulcermin;Ulcogant;
同义名
sucralfate;
基本介绍
A basic aluminum complex of sulfated sucrose. [PubChem]
生产厂家
- Axcan pharma us inc
- Nostrum laboratories inc
- Teva pharmaceuticals usa inc
封装厂家
- Amerisource Health Services Corp.
- Amneal Pharmaceuticals
- A-S Medication Solutions LLC
- Axcan Pharma Inc.
- Bryant Ranch Prepack
- Cardinal Health
- Caremark LLC
- Dept Health Central Pharmacy
- Direct Dispensing Inc.
- Diversified Healthcare Services Inc.
- Eon Labs
- Giant Food Inc.
- Golden State Medical Supply Inc.
- H.J. Harkins Co. Inc.
- Heartland Repack Services LLC
- Ivax Pharmaceuticals
- Levista Inc.
- Long Wing International Inc.
- Major Pharmaceuticals
- Mckesson Corp.
- Medisca Inc.
- Merckle GmbH
- Murfreesboro Pharmaceutical Nursing Supply
- Nostrum Laboratories Inc.
- Nucare Pharmaceuticals Inc.
- PD-Rx Pharmaceuticals Inc.
- Pharmaceutical Association
- Pharmaceutical Utilization Management Program VA Inc.
- Pharmedix
- Physicians Total Care Inc.
- Prasco Labs
- Precision Dose Inc.
- Prepak Systems Inc.
- Prescript Pharmaceuticals
- Prime European Therapeuticals SPA
- Qingdao Pana Life Biochem Co. Ltd.
- Resource Optimization and Innovation LLC
- Sandhills Packaging Inc.
- Sanofi-Aventis Inc.
- Stat Rx Usa
- Teva Pharmaceutical Industries Ltd.
- UDL Laboratories
- Vangard Labs Inc.
- Vistapharm Inc.
- Warrick Pharmaceuticals Corp.
- Watson Pharmaceuticals
- Xactdose Inc.
参考
| Synthesis Reference | Not Available |
| General Reference |
|
剂型
规格
化合物类型
| Type | small molecule |
| Classes | Not Available |
| Substructures | Not Available |
适应症
gastric acid RELATED DISORDERS 中和胃酸;
药理
| Indication | For the short-term treatment (up to 8 weeks) of active duodenal ulcer, as well as maintenance therapy for duodenal ulcer patients at reduced dosage (1 gram twice a day) after healing of acute ulcers. Also used for the short-term treatment of gastric ulcer. |
| Pharmacodynamics | Sucralfate is a prescription medication used to treat peptic ulcers. The current clinical uses of sucralfate are limited. It is effective for the healing of duodenal ulcers, but it is not frequently used for this since more effective drugs (e.g. proton pump inhibitors) have been developed. Although the mechanism of sucralfate's ability to accelerate healing of duodenal ulcers remains to be fully defined, it is known that it exerts its effect through a local, rather than systemic, action. Chemically, sucralfate is a complex of the disaccharide sugar, sucrose, combined with sulfate and aluminum. In acidic solutions (e.g. gastric acid) it forms a thick paste that has a strong negative charge. |
| Mechanism of action | Although sucralfate's mechanism is not entirely understood, there are several factors that most likely contribute to its action. Sucralfate, with its strong negative charge, binds to exposed positively-charged proteins at the base of ulcers. In this way, it coats the ulcer and forms a physical barrier that protects the ulcer surface from further injury by acid and pepsin. It directly inhibits pepsin (an enzyme that breaks apart proteins) in the presence of stomach acid and binds bile salts coming from the liver via the bile thus protecting the stomach lining from injury caused by the bile acids. Sucralfate may increase prostaglandin production. Prostaglandins are known to protect the lining of the stomach and may also bind epithelial growth factor and fibroblast growth factor, both of which enhance the growth and repair mechanism of the stomach lining. |
| Absorption | Minimally absorbed from the gastrointestinal tract (up to 5% of the disaccharide component and less than 0.02% of aluminum). |
| Volume of distribution | Not Available |
| Protein binding | Not Available |
| Metabolism |
Not Available
|
| Route of elimination | The small amounts of the sulfated disaccharide that are absorbed are excreted primarily in the urine. |
| Half life | Not known. |
| Clearance | Not Available |
| Toxicity | Acute oral toxicity (LD50) in mice is >8000 mg/kg. There is limited experience in humans with overdosage of sucralfate. Sucralfate is only minimally absorbed from the gastrointestinal tract and thus risks associated with acute overdosage should be minimal. In rare reports describing sucralfate overdose, most patients remained asymptomatic. |
| Affected organisms |
|
| Pathways | Not Available |
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
||||||||||||||||||||||||||||||||||||||||||
药物相互作用
| Drug | Interaction |
|---|---|
| Calcipotriol | Vitamin D Analogs may increase the serum concentration of Sucralfate. Specifically, the absorption of aluminum from sucralfate may be increased, leading to an increase in the serum aluminum concentration. Avoid chronic and/or excessive use of aluminum and aluminum-containing products (such as sucralfate) in patients who are also taking vitamin D analogs. Any patients consuming such a combination should be monitored closely for aluminum status and signs/symptoms of aluminum-related toxicities. |
| Cholecalciferol | Vitamin D analogs such as cholecalciferol may increase the serum concentration of sucralfate. Specifically, the absorption of aluminum from sucralfate may be increased, leading to an increase in the serum aluminum concentration. Avoid chronic and/or excessive use of aluminum and aluminum-containing products (such as sucralfate) in patients who are also taking vitamin D analogs. Any patients consuming such a combination should be monitored closely for aluminum status and signs/symptoms of aluminum-related toxicities. |
| Ciprofloxacin | Formation of non-absorbable complexes |
| Clodronate | Formation of non-absorbable complexes |
| Etidronic acid | Formation of non-absorbable complexes |
| Fosphenytoin | Sucralfate decreases the effect of hydantoin |
| Gatifloxacin | Formation of non-absorbable complexes |
| Gemifloxacin | Formation of non-absorbable complexes |
| Grepafloxacin | Formation of non-absorbable complexes |
| Ibandronate | Formation of non absorbable complexes |
| Itraconazole | Sucralfate may decrease the absorption of itraconazole. |
| Ketoconazole | Sucralfate may decrease the absorption of ketoconazole. |
| Lansoprazole | Sucralfate decreases the effect of lansoprazole |
| Levofloxacin | Formation of non-absorbable complexes |
| Levothyroxine | Sucralfate decreases the effect of levothyroxine |
| Moxifloxacin | Formation of non-absorbable complexes |
| Norfloxacin | Formation of non-absorbable complexes |
| Ofloxacin | Formation of non-absorbable complexes |
| Phenytoin | Sucralfate decreases the effect of hydantoin |
| Trovafloxacin | Sucralfate may decrease the absorption of orally administered Trovafloxacin. The Sucralfate formulation contains aluminum ions that may intefere with Trovafloxacin absorption. Administer Trovafloxacin 2 hours before or 6 hours after the Sucralfate dose to minimize the interaction. |
| Warfarin | Sucralfate may reduce the absorption of warfarin. Warfarin should be administered at least 2 hours before or 6 hours after sucralfate administration. Monitor for changes in prothrombin time if sucralfate is initiated, discontinued or dose changed. |
食物相互作用
- Avoid alcohol.
- Do not take calcium, aluminum, magnesium or Iron supplements within 2 hours of taking this medication.
- Take on empty stomach: 1 hour before or 2 hours after meals.
- Take with a full glass of water.