药品详细
Cefmenoxime(头孢甲肟)
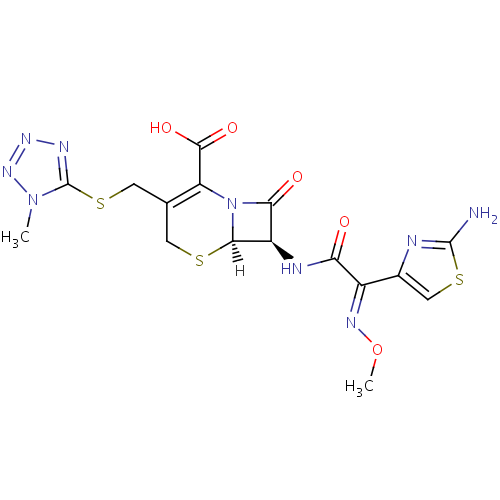
化学结构式图
中文名
头孢甲肟
英文名
Cefmenoxime
分子式
C16H17N9O5S3
化学名
(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-3-{[(1-methyl-1H-1,2,3,4-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
分子量
Average: 511.558
Monoisotopic: 511.051476769
Monoisotopic: 511.051476769
CAS号
65085-01-0
ATC分类
J01D Other Beta- lactam Antibacterials
药物类型
small molecule
阶段
approved
商品名
Bestcall;Cefmax;Tacef;
同义名
Cefmenoxima [INN-Spanish];Cefmenoxime hydrochloride;Cefmenoximum [INN-Latin];
基本介绍
Cefmenoxime is a third-generation cephalosporin antibiotic. [Wikipedia]
生产厂家
- Tap pharmaceutical products inc
封装厂家
参考
| Synthesis Reference | Not Available |
| General Reference |
|
剂型
规格
化合物类型
| Type | small molecule |
| Classes | Not Available |
| Substructures | Not Available |
适应症
antibacterials 抗细菌;
药理
| Indication | Used to treat female gynecologic and obstetric infections caused by susceptible aerobic (including the gonococcus) and anaerobic bacteria. |
| Pharmacodynamics | Cefmenoxime is a semisynthetic beta-lactam cephalosporin antibiotic with activity similar to that of cefotaxime. It has broad spectrum activity against Gram positive and Gram negative bacteria. |
| Mechanism of action | The bactericidal activity of cefmenoxime results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs). Cefmenoxime is stable in the presence of a variety of b-lactamases, including penicillinases and some cephalosporinases. |
| Absorption | Bioavailability is approximately 100% following intramuscular injection. |
| Volume of distribution | Not Available |
| Protein binding | 50-70% |
| Metabolism |
Not appreciably metabolized.
|
| Route of elimination | Not Available |
| Half life | 1 hour |
| Clearance | Not Available |
| Toxicity | Information on cefmenoxime overdosage in humans is not available. However, with other b-lactam antibiotics, adverse effects following overdosage have included nausea, vomiting, epigastric distress, diarrhea, and convulsions. |
| Affected organisms |
|
| Pathways | Not Available |
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
||||||||||||||||||||||||||||||||||||||||||
药物相互作用
食物相互作用
Not Available