药品详细
Cefmetazole(头孢美唑)
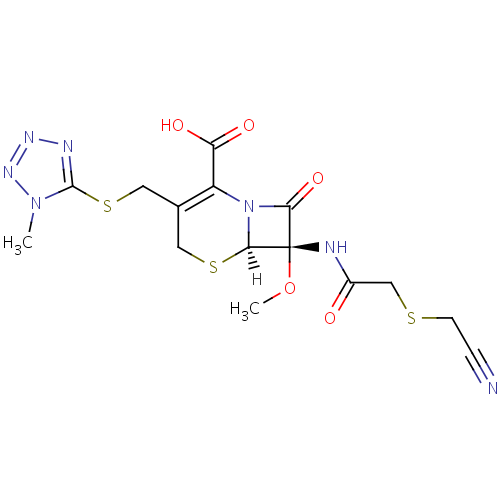
化学结构式图
中文名
头孢美唑
英文名
Cefmetazole
分子式
C15H17N7O5S3
化学名
(6R,7S)-7-{2-[(cyanomethyl)sulfanyl]acetamido}-7-methoxy-3-{[(1-methyl-1H-1,2,3,4-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
分子量
Average: 471.534
Monoisotopic: 471.045328759
Monoisotopic: 471.045328759
CAS号
56796-20-4
ATC分类
J01D Other Beta- lactam Antibacterials
药物类型
small molecule
阶段
approved
商品名
Zefazone;
同义名
Cefmetazole sodium;Cefmetazolo [INN-Spanish];Cefmetazolum [INN-Latin];
基本介绍
A semisynthetic cephamycin antibiotic with a broad spectrum of activity against both gram-positive and gram-negative microorganisms. It has a high rate of efficacy in many types of infection and to date no severe side effects have been noted. [PubChem]
生产厂家
- Pharmacia and upjohn co
封装厂家
参考
| Synthesis Reference | Not Available |
| General Reference | Not Available |
剂型
规格
化合物类型
| Type | small molecule |
| Classes |
|
| Substructures |
|
适应症
antibacterials 抗细菌;
药理
| Indication | For the treatment of infections caused by susceptible organisms. |
| Pharmacodynamics | Cefmetazole is a second-generation cephalosporin. The cephalosporins are bactericidal drugs with both gram-positive and gram-negative activity. They inhibit bacterial cell wall synthesis in a way similar to the penicillins. Cefmetazole is more active than 1st-generation cephalosporins against indole-positive Proteus, Serratia, anaerobic gram-negative bacilli (including B. fragilis), and some E. coli, Klebsiella, and P. mirabilis, but is less active than cefoxitin or cefotetan against most gram-negative bacilli. |
| Mechanism of action | The bactericidal activity of cefmetazole results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs). |
| Absorption | Bioavailability is approximately 100% following intramuscular injection. |
| Volume of distribution | Not Available |
| Protein binding | Not Available |
| Metabolism |
No appreciable metabolism.
|
| Route of elimination | Not Available |
| Half life | 1.50 ±0.14 hours |
| Clearance | Not Available |
| Toxicity | Oral LD50 in rats is 3,204 mg/kg. With other b-lactam antibiotics, adverse effects following overdosage have included nausea, vomiting, epigastric distress, diarrhea, and convulsions. |
| Affected organisms |
|
| Pathways | Not Available |
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
||||||||||||||||||||||||||||||||||||||||||
药物相互作用
| Drug | Interaction |
|---|---|
| Probenecid | Probenecid may increase the serum level of cefmatzole. |
食物相互作用
Not Available