药品详细
Aztreonam(氨曲南)
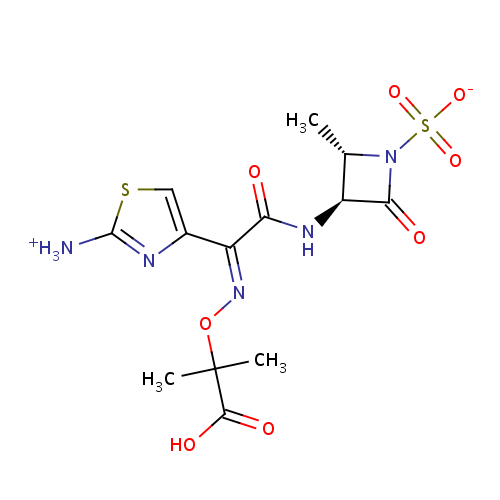
化学结构式图
中文名
氨曲南
英文名
Aztreonam
分子式
C13H17N5O8S2
化学名
(2S,3S)-3-[(2Z)-2-(2-azaniumyl-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]acetamido]-2-methyl-4-oxoazetidine-1-sulfonate
分子量
Average: 435.433
Monoisotopic: 435.051853925
Monoisotopic: 435.051853925
CAS号
78110-38-0
ATC分类
J01D Other Beta- lactam Antibacterials
药物类型
small molecule
阶段
approved
商品名
Azactam;Corus 1020;Dynabiotic;Monobactam;Primbactam;
同义名
AZT;
基本介绍
A monocyclic beta-lactam antibiotic originally isolated from Chromobacterium violaceum. It is resistant to beta-lactamases and is used in gram-negative infections, especially of the meninges, bladder, and kidneys. It may cause a superinfection with gram-positive organisms. [PubChem]
生产厂家
- App pharmaceuticals llc
- Bristol myers squibb
- Bristol myers squibb co
- Gilead sciences inc
封装厂家
参考
| Synthesis Reference | Not Available |
| General Reference | Not Available |
剂型
规格
化合物类型
| Type | small molecule |
| Classes | Not Available |
| Substructures | Not Available |
适应症
antibacterials 抗细菌;
药理
| Indication | For the treatment of the following infections caused by susceptible gram-negative microorganisms: urinary tract infections, lower respiratory tract infections, septicemia, skin and skin-structure infections, intra-abdominal infections, and gynecologic infections. |
| Pharmacodynamics | Aztreonam is a monocyclic beta-lactam antibiotic (a monobactam) originally isolated from Chromobacterium violaceum. Aztreonam exhibits potent and specific activity in vitro against a wide spectrum of gram-negative aerobic pathogens including Pseudomonas aeruginosa. It has no useful activity against gram-positive bacteria or anaerobes, but has very broad spectrum against gram-negative aerobes, including Pseudomonas aeruginosa. This has given it the nickname "the magic bullet for aerobic gram-negative bacteria". Aztreonam, unlike the majority of beta-lactam antibiotics, does not induce beta-lactamase activity and its molecular structure confers a high degree of resistance to hydrolysis by beta-lactamases (such as penicillinases and cephalosporinases) produced by most gram-negative and gram-positive pathogens; it is, therefore, usually active against gram-negative aerobic microorganisms that are resistant to antibiotics hydrolyzed by beta-lactamases. It is active against many strains that are multiply-resistant to other antibiotics, such as certain cephalosporins, penicillin, and aminoglycosides. Aztreonam maintains its antimicrobial activity over a pH range of 6 to 8 in vitro, as well as in the presence of human serum and under anaerobic conditions. |
| Mechanism of action | The bactericidal action of aztreonam results from the inhibition of bacterial cell wall synthesis due to a high affinity of aztreonam for penicillin binding protein 3 (PBP3). By binding to PBP3, aztreonam inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins. It is possible that aztreonam interferes with an autolysin inhibitor. |
| Absorption | Less than 1% absorbed from the gastrointestinal tract following oral administration. Completely absorbed following intramuscular administration. |
| Volume of distribution |
|
| Protein binding | Serum protein binding averaged 56% and is independent of dose. Impaired renal function, 36 to 43%. |
| Metabolism |
Approximately 6 to 16% metabolized to inactive metabolites by hydrolysis of the beta-lactam bond, resulting in an open-ring compound.
|
| Route of elimination | In healthy subjects, aztreonam is excreted in the urine about equally by active tubular secretion and glomerular filtration. Urinary excretion of a single parenteral dose was essentially complete by 12 hours after injection. |
| Half life | The serum half-life of aztreonam averaged 1.7 hours (1.5 to 2.0) in subjects with normal renal function, independent of the dose. In elderly patients and in patients with impaired renal function, the mean serum half-life of aztreonam increased (4.7 to 6 hours and 2.1 hours, respectively). |
| Clearance |
|
| Toxicity | Not Available |
| Affected organisms |
|
| Pathways | Not Available |
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
||||||||||||||||||||||||||||||||||||||||||
药物相互作用
| Drug | Interaction |
|---|---|
| Demeclocycline | Possible antagonism of action |
| Doxycycline | Possible antagonism of action |
| Ethinyl Estradiol | This anti-infectious agent could decrease the effect of the oral contraceptive |
| Minocycline | Possible antagonism of action |
| Tetracycline | Possible antagonism of action |
食物相互作用
Not Available