药品详细
Entecavir (恩替卡韦 )
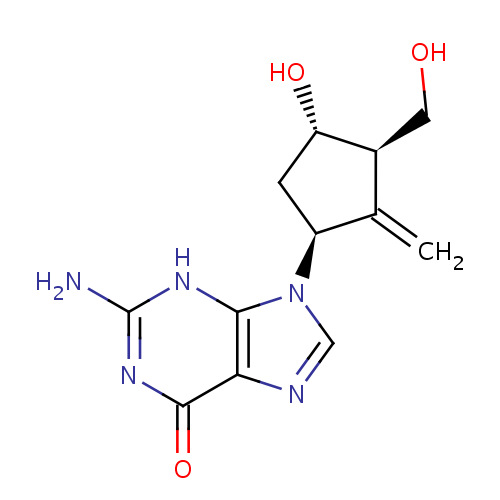
化学结构式图
中文名
恩替卡韦
英文名
Entecavir
分子式
Not Available
化学名
2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-6,9-dihydro-3H-purin-6-one
分子量
Average: 277.2792
Monoisotopic: 277.117489371
Monoisotopic: 277.117489371
CAS号
142217-69-4
ATC分类
J05A Direct acting antivirals
药物类型
small molecule
阶段
商品名
Baraclude;
同义名
entecavir;
基本介绍
Entecavir is an oral antiviral drug used in the treatment of hepatitis B infection. It is marketed under the trade name Baraclude (BMS).
Entecavir is a guanine analogue that inhibits all three steps in the viral replication process, and the manufacturer claims that it is more efficacious than previous agents used to treat hepatitis B (lamivudine and adefovir). It was approved by the U.S. Food and Drug Administration (FDA) in March 2005.
生产厂家
- Bristol myers squibb
封装厂家
参考
| Synthesis Reference | Not Available |
| General Reference | Not Available |
剂型
| Form | Route | Strength |
|---|---|---|
| Tablet | Oral |
规格
| Unit description | Cost | Unit |
|---|---|---|
| Baraclude 0.5 mg tablet | 28.94 USD | tablet |
| Baraclude 1 mg tablet | 28.94 USD | tablet |
化合物类型
| Type | small molecule |
| Classes |
|
| Substructures |
|
适应症
ANTIVIRALS 抗病毒;
药理
| Indication | For the treatment of chronic hepatitis B virus infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. |
| Pharmacodynamics | Entecavir is a guanosine nucleoside analogue with selective activity against hepatitis B virus (HBV). It is designed to selectively inhibit the Hepatitis B virus, blocking all three steps in the replication process. Entecavir is more efficient than an older Hepatitis B drug, lamivudine. |
| Mechanism of action | By competing with the natural substrate deoxyguanosine triphosphate, entecavir functionally inhibits all three activities of the HBV polymerase (reverse transcriptase, rt): (1) base priming, (2) reverse transcription of the negative strand from the pregenomic messenger RNA, and (3) synthesis of the positive strand of HBV DNA. Upon activation by kinases, the drug can be incorporated into the DNA which has the ultimate effect of inhibiting the HBV polymerase activity. |
| Absorption | Absorption Following oral administration in healthy subjects, entecavir peak plasma concentrations occurred between 0.5 and 1.5 hours. In healthy subjects, the bioavailability of the tablet is 100% relative to the oral solution. |
| Volume of distribution | Not Available |
| Protein binding | Binding of entecavir to human serum proteins in vitro is approximately 13%. |
| Metabolism |
Entecavir is not a substrate, inhibitor, or inducer of the cytochrome P450 (CYP450) enzyme system. Entecavir is efficiently phosphorylated to the active triphosphate form. |
| Route of elimination | Not Available |
| Half life | After reaching peak concentration, entecavir plasma concentrations decreased in a bi-exponential manner with a terminal elimination half-life of approximately 128-149 hours. The phosphorylated metabolite has a half-life of 15 hours. |
| Clearance |
|
| Toxicity | Healthy subjects who received single entecavir doses up to 40 mg or multiple doses up to 20 mg/day for up to 14 days had no increase in or unexpected adverse events. If overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary. |
| Affected organisms |
|
| Pathways | Not Available |
理化性质
| Properties | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | solid | ||||||||||||||||||||||||||||||||||||
| Melting point | Not Available | ||||||||||||||||||||||||||||||||||||
| Experimental Properties |
|
||||||||||||||||||||||||||||||||||||
| Predicted Properties |
|
||||||||||||||||||||||||||||||||||||
药物相互作用
食物相互作用
- Take on an empty stomach.
- Taking the product with a high-fat meal or a light snack reduces the maximal concentration by 44 to 46% and total exposure by 18 to 20%.